Celavet Clinical Trials
Celavie Biosciences and subsidiary Celavet are developing therapies using undifferentiated allogeneic pluripotent stem cells to repair damaged tissues and restore their functions. Celavie and Celavet produce large banks of undifferentiated cells across 4 mammalian lines that are grown in a specialized patented medium allowing uniform qualities and genetic stabilities. Research in one area leads to innovation in another. Early trials have demonstrated safety and the promise of efficacy.
Multicenter Open Label Study of OK100 Stem Cells for Treatment of Equine Tendon Injuries
Double Blind Randomized Trial of OK100 Stem Cells for Treatment of Acute Equine Tendinitis Lesions
Our equine OK100 stem cells were successfully tested in a double-blind, controlled study at Cornell University’s Large Animal Lab by Principal Investigator Dr. Alan Nixon, DVM, BVSc, MS.
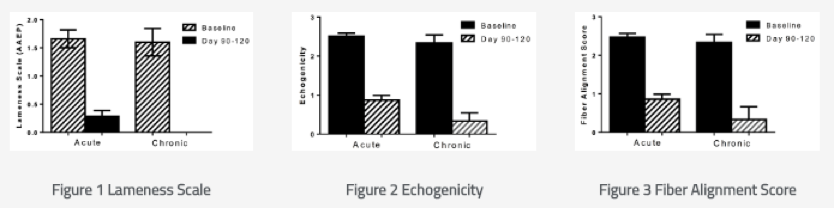
Ultrasonographical and morphological results showed:
- Complete reconstruction of the experimental lesion
- Anatomically correct fiber alignment as shown by ultrasound
- Progressive healing in ESC injected tendons
- Persisting echolucent core lesion in the control group
- Increasing deterioration of lesion in the control group
- All horses injected with ESCs demonstrating ultrasonographic evidence of improvement in healing
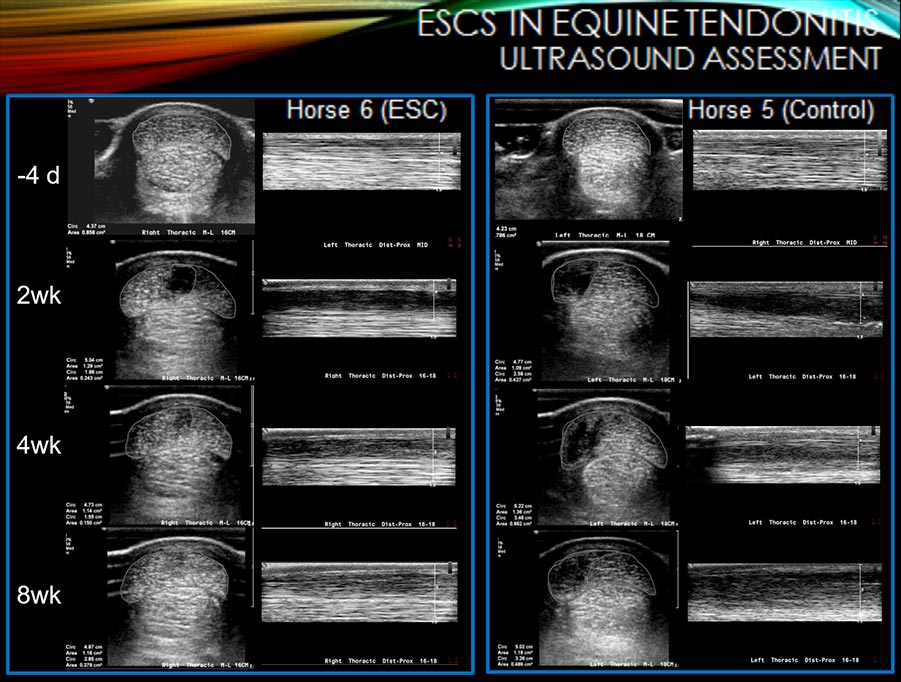
The figure above shows examples of the ultrasonographic healing progression between Horse 6 (ESC, treated with equine undifferentiated stem cells OK100) and Horse 5 (Control-sham injection). (Watts A, Yeager A, Kopyov O, Nixon A. Fetal derived embryonic-like stem cells improve healing in a large animal flexor tendonitis model. Stem Cell Research & Therapy. 2011 Jan 27;2(1):4.)
As a part of evaluating safety of OK99 stem cells for human use, Celavie Biosciences contracted Seventh Wave Laboratories, LLC (Chesterfield, MO) to perform a study, “Does Intraparenchymal Injection of Canine Allogeneic Stem Cells into the Canine Brain Elicit Immune Response.” To study the immunogenicity of Celavie stem cells in the same species environment, our canine stem cells were transplanted into the brains of healthy beagles. The blood and brain tissue were examined for signs of immune reaction to the cells. No immune response to OK99 stem cells was detected.
To further explore canine treatments, the following pilot study under IND OK200 was conducted: “An Open Label Study to Test the Safety and Efficacy of Fetal-derived Allogeneic Canine Stem Cells for the Treatment of Chronic Osteoarthritis and Cruciate Ligament Injuries in Canines.”
IND 012-003 was issued in January 2011, in which:
- Eight dogs with chronic osteoarthritis were injected intravenously
- In 12 dogs, the cells were injected directly into the joints
- No tumor formation or infection was detected
The study was put on hold to concentrate efforts on the human and equine project.
Case Study: Wounds and Burns
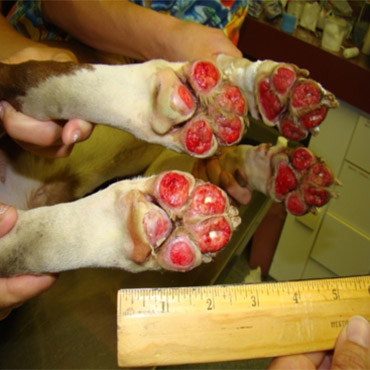
Pre Surgery – July 26, 2011
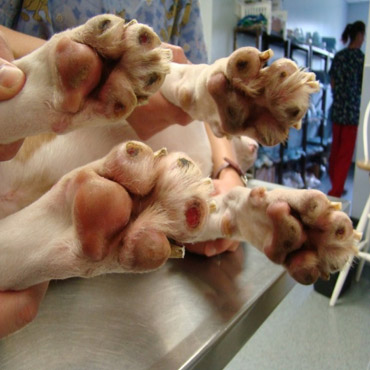
Post Surgical – September 13, 2011
Bernie was an abused pit bull whose owner left him on the metal roof of a row home in Reading, PA for several scorching hot summer days during which temperatures reached 110°F. He developed severe third-degree burns to all four paws (no skin remaining), as well as burns to his back and legs. The local director of the Animal Rescue League came to Celavet because of our pilots with stem cells and asked us to help this animal.
After CVM approved compassionate use treatment, Dr. Wagner of Wyomissing Animal Hospital and Dr. Kopyov of Celavet, came up with a technique that involved injection of the cells in a circular pattern around the rim of each pad, and then applied scaffolding to hold the cells in place and give them a surface for attachment. The paws were then wrapped and bandaged. Dr. Wagner observed: “almost total restoration, no scar tissue in 5 weeks.”
Celavet is committed to a preclinical study and submitting an Investigational New Animal Drug (INAD) for chronic wounds and burns.