Celavie Biosciences Clinical Trials
Celavie Biosciences and subsidiary Celavet are developing therapies using undifferentiated allogeneic pluripotent stem cells to repair damaged tissues and restore their functions. Celavie and Celavet produce large banks of undifferentiated cells across 4 mammalian lines that are grown in a specialized patented medium allowing uniform qualities and genetic stability. Research in one area leads to innovation in another. Early trials have demonstrated safety and the promise of efficacy.
The first-in-human exploratory study of Celavie stem cells for Parkinson’s Disease in Mexico
-
- Purpose: To evaluate the safety of OK99 stem cells in treating patients suffering from Parkinson’s disease
- Conduct: Hospital Angeles del Pedregal, Mexico City. Study approved by the Federal Commission for Protection against Sanitary Risks (COFEPRIS – Mexico’s FDA equivalent)
- Methodology: 8 patients with moderate to severe PD not adequately controlled with medication were selected for the study. OK99 stem cells were injected into putamen. Patients were immunosuppressed with Cyclosporine for one month after injection. Patients were examined with Positron Emission Tomography (PET) using 3 radio markers (two per patient) to fully evaluate relevant molecular changes in the brain after transplantation. The patients were divided into 2 groups: one was given dihidrotetrabenazine (DTBZ) and raclopride; and the other DTBZ and fluoro-L-DOPA.
- Time frame: Five year follow-up is completed
- Results: Celavie presented poster, “Five year follow-up on the first-in-human transplantation of undifferentiated stem cells into Parkinsonian patients reveals no adverse effects with improvement in motor function or arrest of the disease progression in five out of seven patients,” at the Phacilitate Leaders World and World Stem Cell Summit, Jan. 2020. Four year results published in Cell Transplantation, Nov. 2018. Follow-up revealed no tumors, no immune rejection and no lasting severe adverse events. Unified Parkinson’s Disease Rating Scale (UPDRS) III scores demonstrated arrest and in some patients reversal of the progression of Parkinson’s Disease.
OK99 Stem Cells in Development to Treat Parkinson’s Disease
Based on the positive safety and functional results of the five year follow up of the first-in-human exploratory study, Celavie is in the process of obtaining FDA approval for Phase I clinical study of OK99 for Parkinson’s Disease in US.
OK99 Stem Cells, Exploratory the First-in-Human Clinical Study, Mexico
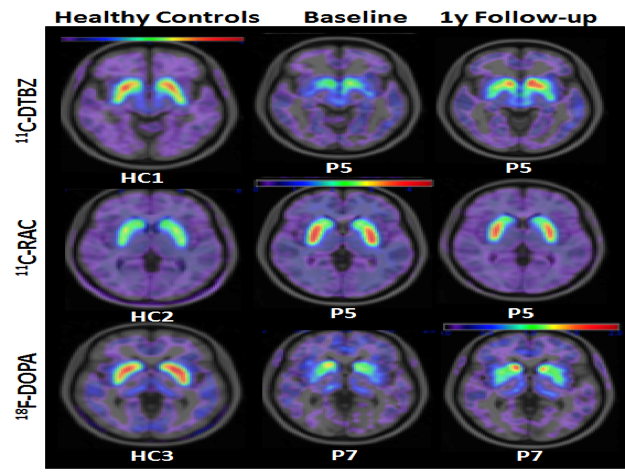
Top row: A larger area of the brain 1 year after transplantation (colored in red) documents increased release of dopamine into the specific area of the brain that directly correlates with clinical improvement in PD in a sample patient.
Middle row: A smaller area of the brain 1 year after transplantation (colored in red) documents that a selective antagonist of dopamine, RAC, occupies a smaller area because of the increased competition from increased levels of dopamine. This correlates with clinical improvement in PD.
Bottom row: A larger area of the brain 1 year after transplantation documents increased production of dopamine in the brain, which directly correlates with clinical improvement in PD.
- Key:
- 11C-DTBZ, marker for vesicular monoamine transporter 2 (VMAT2) activity
- 11C-RAC , marker for dopaminergic (D2) receptor density
- 18F-DOPA reflects level of dopamine synthesis.
- Higher DTBZ and F-DOPA uptake and lower RAC uptake together reflect increased dopamine production, which might underlie the patients’ improved scorings on the UPDRS.
- Key:
- Status: Five year follow-up completed
- Pilot study in 7 PD patients with 5-year follow-up completed and results have been published
- In the process of obtaining FDA IND approval for conducting larger controlled double-blinded randomized trial in US
- Publication:
The results of four year follow up are published in “Cell Transplantation”. The study is registered at #ISRCTN39104513
The five year results were presented at the Phacilitate Leaders World and World Stem Cell Summit, Jan. 2020. - Learn More:
Download “Transplantation of Human Neural Progenitor Cells (NPC) into Putamina of Parkinsonian Patients: A Case Series Study, Safety and Efficacy Four Years after Surgery”
Download “Five year follow-up on the first-in-human transplantation of undifferentiated stem cells into Parkinsonian patients reveals no adverse effects with improvement in motor function or arrest of the disease progression in five out of seven patients”
Phase 1 Double-blind Placebo Controlled Randomized Trial in US. Application to FDA is in Progress.
- Purpose: To evaluate the safety of Celavie’s stem cells in treating patients suffering from Parkinson’s disease
- Conduct: Hoag Memorial Hospital Presbyterian, Newport Beach, CA
- Time frame: 3 years
- Methodology: This will be a two-part study, wherein Part I is a staggered-start open label longitudinal trial, and Part II is a randomized, two arm, double-blind, controlled, longitudinal trial of Celavie stem cells for the treatment of moderate to advanced Parkinson’s Disease. Stage II will be launched only if 3 month follow up of the patients enrolled and treated in Stage I reveals no permanent, serious adverse events due to treatment. The primary end measure is safety: number and severity of side effects and complications. The secondary end measures include variable neurological, neuropsychological and radiological evaluations (Positron Emission Tomography – PET) established in Core Assessment Program for Intracerebral Transplantations (CAPIT). Evaluations will be performed during on and off medication conditions at baseline, 6 months, 1 and 2 years after transplantation. The neurosurgical facility has state-of-the-art stereotactic equipment, CAT scan, MRI and Positron Emission Tomography.
- Status: To be scheduled
- Filed for pre-IND guidance for trial with FDA.
- Dr. Oleg Kopyov and Dr. Christopher Duma designing protocol.



